Certification: Corrosion and Materials
Certification Full Name: Corrosion and Materials
Certification Provider: API
Exam Code: API-571
Exam Name: Corrosion and Materials
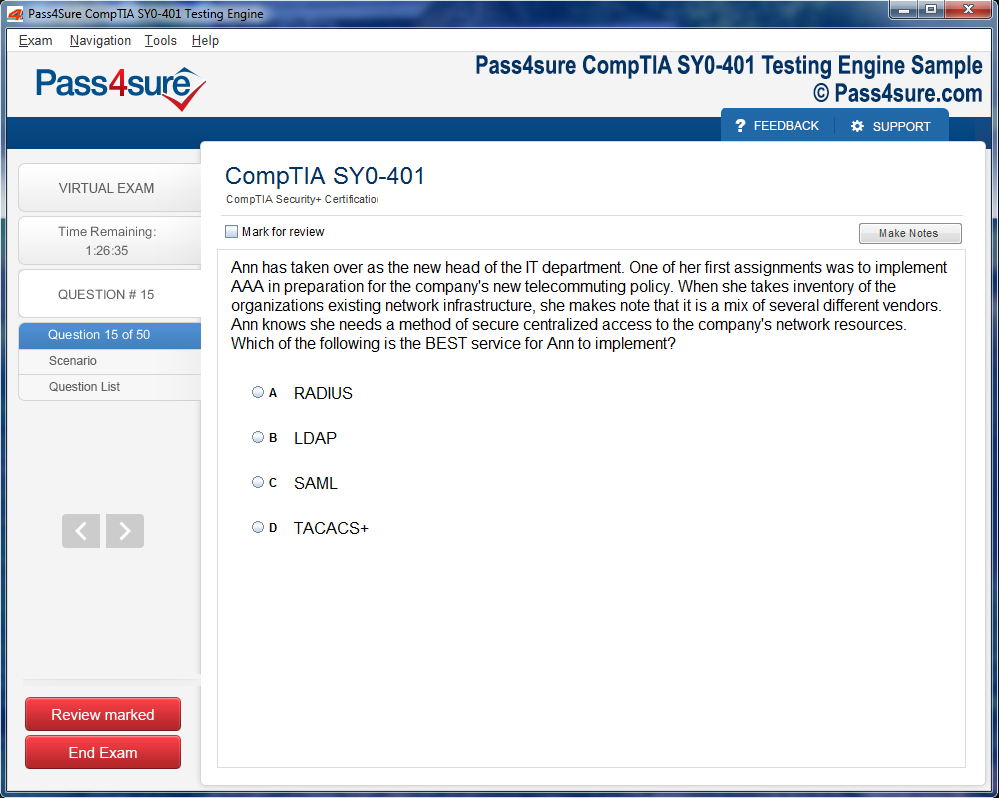
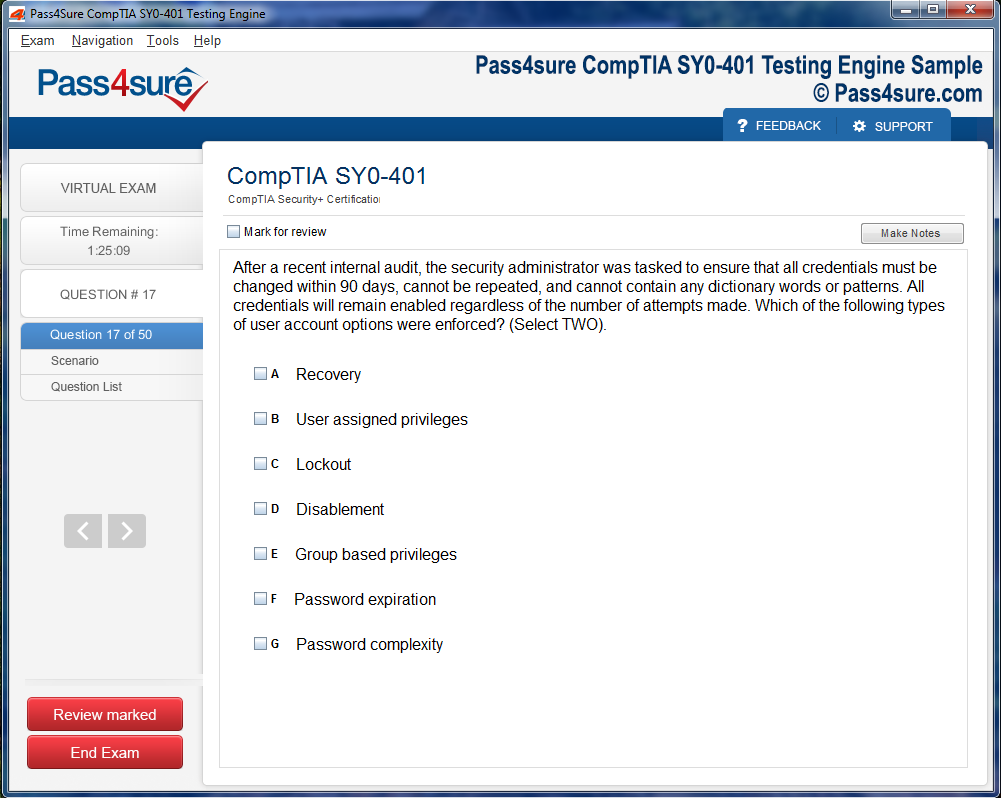
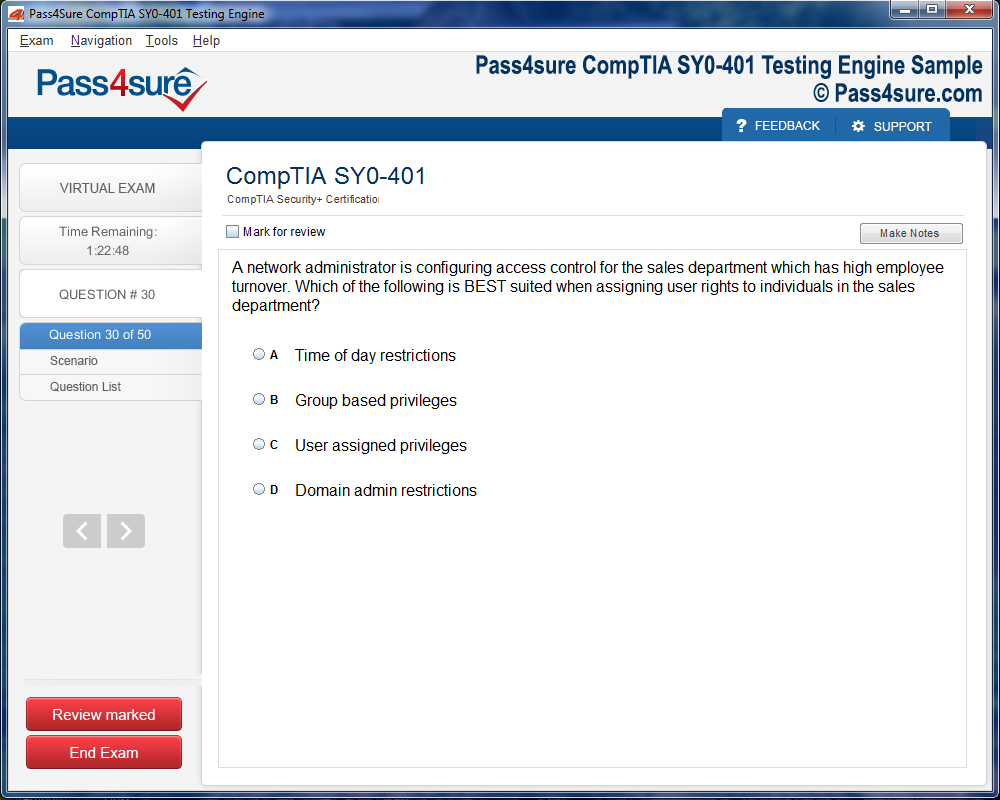
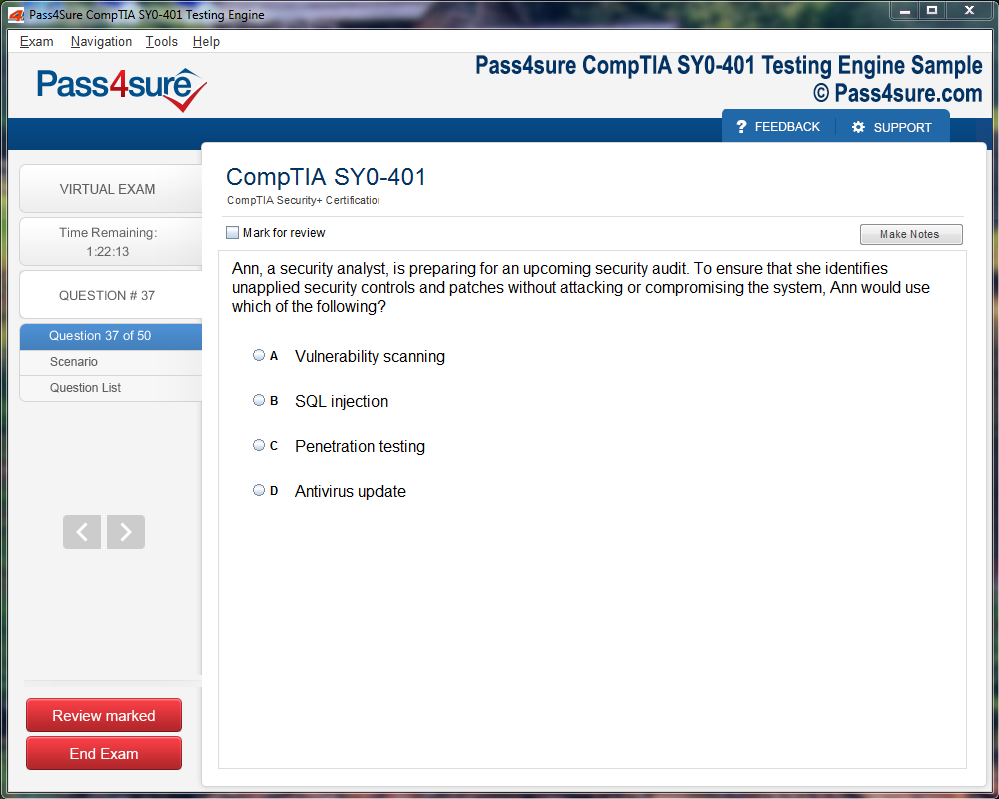
Product Screenshots
The Beginner's Guide to API Corrosion and Materials Certification
Corrosion is a natural, inevitable process, but its impact on materials can be both alarming and costly. To understand why corrosion occurs and why it affects materials so dramatically, we must explore the science behind it. At its core, corrosion is the result of chemical reactions between a material, typically metal, and its surrounding environment. These reactions degrade the material, often compromising its mechanical properties and structural integrity.
In most cases, corrosion is initiated when a metal comes into contact with elements like water, oxygen, and sometimes acidic substances. This process typically begins at the atomic level, where the metal atoms lose electrons and transform into positively charged ions. These ions then bond with other substances in the environment, forming compounds such as rust or scale on the surface. The rate of corrosion can vary significantly depending on factors like the type of material, the chemical makeup of the environment, and the temperature.
A critical aspect of corrosion is its ability to spread and worsen over time. What starts as a small, localized pit or crack can eventually lead to large-scale damage if not addressed. This is particularly concerning in industries that rely on heavy machinery and structural components, such as oil and gas, where the failure of a component due to corrosion can have devastating consequences. The reason corrosion is so dangerous is that it often occurs silently, beneath the surface, making it difficult to detect until it has reached an advanced stage.
Understanding the various forms of corrosion is essential for industries that work with metals and other materials prone to degradation. Common types of corrosion include uniform corrosion, galvanic corrosion, pitting corrosion, and stress corrosion cracking. Each of these forms has different characteristics and can be triggered by different environmental conditions. For example, pitting corrosion occurs when small, localized areas on a metal surface are attacked, often creating holes that can grow and worsen over time. Stress corrosion cracking, on the other hand, is caused by the combination of tensile stress and a corrosive environment, leading to the formation of cracks that can ultimately cause a catastrophic failure.
The impact of corrosion on materials can be profound. Not only does it compromise the strength and durability of materials, but it can also lead to unexpected failures that result in costly downtime and repairs. In industries like oil and gas, where equipment is often exposed to corrosive substances like seawater, hydrogen sulfide, and sulfur compounds, the consequences of corrosion can be disastrous. This is why it is critical to implement preventive measures, such as the use of corrosion-resistant materials and the adoption of robust corrosion management practices.
Materials that are particularly susceptible to corrosion, such as carbon steel and mild steel, are commonly used in a wide range of industrial applications. However, when exposed to corrosive environments, these materials degrade rapidly, often requiring frequent maintenance and replacement. To mitigate the risks associated with corrosion, industries rely on advanced materials that are specifically designed to withstand these harsh conditions. These materials, often made from alloys or treated metals, are engineered to resist the damaging effects of corrosion, prolonging the lifespan of equipment and reducing the overall cost of maintenance.
This is where API corrosion and material certification play a pivotal role. The standards set forth by the American Petroleum Institute help ensure that materials used in the oil and gas industry are carefully selected and tested for their corrosion resistance. By adhering to these standards, companies can significantly reduce the likelihood of equipment failure, improve safety, and minimize the environmental impact of corrosion-related incidents.
The Role of API Standards in Material Selection
The American Petroleum Institute (API) is a leading authority in the development of standards that govern the materials used in the oil and gas industry. API standards are designed to ensure that equipment and materials meet specific requirements for safety, performance, and durability, with a strong emphasis on corrosion resistance. The organization has established a comprehensive set of guidelines that cover everything from the selection of raw materials to the testing and certification of finished products.
API standards are crucial for industries that operate in corrosive environments because they provide a framework for selecting materials that are most suitable for the task at hand. The process of material selection is complex and involves evaluating various factors, including the specific type of corrosion that may be encountered, the mechanical properties required for the application, and the environmental conditions that will affect the material over time. By following API standards, companies can ensure that they are using the best possible materials for their applications, thereby improving the longevity and reliability of their equipment.
One of the key elements of API material certification is the rigorous testing that materials undergo before they are approved for use in critical applications. These tests are designed to simulate the harsh conditions that materials will face during their service life. For example, materials may be subjected to accelerated corrosion tests, where they are exposed to extreme temperatures, high-pressure environments, or corrosive chemicals. The results of these tests help determine whether a material is suitable for a particular application and if it meets the required performance standards.
API certification also ensures that materials used in the oil and gas industry are compatible with other materials in the system. In many cases, different metals and alloys are used in the same system, which can create the potential for galvanic corrosion. This occurs when two dissimilar metals come into contact with each other in the presence of an electrolyte, leading to the corrosion of one of the metals. API standards address this issue by specifying the appropriate combinations of materials to prevent galvanic corrosion and ensure the overall integrity of the system.
Furthermore, API standards are designed to ensure that materials are tested for their resistance to various forms of corrosion, including pitting, crevice corrosion, and stress corrosion cracking. These types of corrosion can be particularly damaging to equipment, leading to the formation of holes, cracks, or fractures that can compromise the strength and safety of the material. By adhering to API certification requirements, companies can reduce the risk of these types of corrosion, ultimately improving the reliability and safety of their equipment.
In addition to corrosion resistance, API standards also consider other factors that impact material performance, such as toughness, strength, and fatigue resistance. These properties are essential for ensuring that materials can withstand the mechanical stresses and strains encountered during normal operation, as well as during extreme events such as pressure surges or temperature fluctuations. API-certified materials are designed to perform reliably under a wide range of conditions, ensuring that they will not fail prematurely and will continue to function effectively over time.
Part 4: The Impact of Environmental Factors on Corrosion
Environmental factors play a significant role in the rate and severity of corrosion. The presence of water, oxygen, and other chemicals in the environment can accelerate the corrosion process, leading to more rapid degradation of materials. Understanding these environmental influences is crucial for selecting the right materials for specific applications and for implementing effective corrosion prevention measures.
One of the primary environmental factors that contributes to corrosion is moisture. Water, particularly when combined with oxygen, acts as an electrolyte that facilitates the transfer of electrons between the material and the environment, speeding up the corrosion process. This is why materials exposed to humid or wet conditions are more likely to corrode than those in dry environments. For example, pipelines and storage tanks used in the oil and gas industry are often exposed to rain, seawater, or even underground moisture, all of which can significantly accelerate corrosion.
In addition to moisture, the presence of salts and other corrosive chemicals can further exacerbate the corrosion process. Seawater, for instance, contains high concentrations of chloride ions, which can cause pitting and crevice corrosion in metals. Similarly, sulfur compounds, commonly found in oil and gas fields, can lead to the formation of sulfuric acid, which can rapidly degrade materials. These environmental challenges highlight the need for materials that are specifically designed to withstand such corrosive conditions.
Temperature is another critical factor that affects the rate of corrosion. In general, higher temperatures tend to increase the rate of corrosion by accelerating the chemical reactions that cause degradation. For example, materials exposed to high temperatures in offshore oil platforms or refineries may experience more rapid corrosion than those in cooler environments. Temperature fluctuations, such as those caused by seasonal changes or operational cycles, can also contribute to the formation of cracks or other forms of damage.
Pressure is yet another environmental factor that can influence the corrosion process. High-pressure environments, such as those encountered in deepwater drilling or gas pipelines, can cause stress corrosion cracking, a particularly dangerous form of degradation. This occurs when a material is subjected to both tensile stress and a corrosive environment, causing cracks to form that can grow over time and eventually lead to failure. Materials used in high-pressure applications must therefore be carefully selected to resist this type of corrosion.
Part 5: Corrosion Prevention and Management Strategies
Given the significant risks associated with corrosion, it is essential to implement effective prevention and management strategies to minimize its impact. Corrosion prevention strategies can be broadly classified into three categories: material selection, protective coatings, and environmental control. Each of these strategies plays a critical role in ensuring the longevity and reliability of equipment exposed to corrosive environments.
The most fundamental approach to corrosion prevention is selecting the right materials for the job. As discussed earlier, materials that are resistant to corrosion are critical in industries like oil and gas, where equipment is constantly exposed to harsh environments. Corrosion-resistant materials, such as stainless steel, alloy steels, and non-metallic composites, are commonly used in applications where corrosion is a major concern. These materials are designed to resist the effects of moisture, chemicals, and high temperatures, ensuring that they can withstand the corrosive forces they encounter.
In some cases, protective coatings can be applied to materials to create a barrier that prevents corrosion from occurring. These coatings are often used in conjunction with corrosion-resistant materials to provide an additional layer of protection. Common types of protective coatings include paints, galvanization, and anodizing, all of which help to prevent moisture and other corrosive substances from coming into direct contact with the underlying material. In addition to providing corrosion resistance, these coatings can also enhance the aesthetic appearance of equipment and components.
Another important aspect of corrosion management is environmental control. By reducing the exposure of equipment to corrosive substances, the rate of corrosion can be slowed significantly. For example, reducing the amount of moisture in the environment or controlling the concentration of corrosive chemicals can help prevent or minimize corrosion. In some cases, the use of inhibitors or corrosion-resistant additives can be used to reduce the corrosive effects of certain chemicals, providing further protection for materials.
Finally, regular inspection and maintenance are essential for identifying and addressing corrosion before it leads to failure. Routine inspections allow operators to detect early signs of corrosion, such as surface pitting or discoloration, and take corrective actions before the damage becomes severe. In some cases, materials may need to be replaced or repaired, but early detection and intervention can help prevent costly downtime and improve the overall safety of the operation.
The Future of Corrosion Resistance and Material Certification
The future of corrosion resistance and material certification looks promising, with ongoing advancements in materials science and technology offering new solutions for combating corrosion. Researchers are continually developing new alloys and coatings that offer superior resistance to corrosion, while also improving other material properties such as strength and durability. These innovations hold the potential to revolutionize industries that rely heavily on materials exposed to harsh conditions, offering more reliable and cost-effective solutions for preventing corrosion.
One area of active research is the development of self-healing materials, which are capable of repairing themselves when damaged by corrosion. These materials are designed to detect the formation of cracks or pits and initiate a healing process to restore the material’s integrity. While self-healing materials are still in the early stages of development, they offer exciting possibilities for reducing the long-term costs and risks associated with corrosion.
In addition to advancements in materials, the field of corrosion monitoring is also evolving. New technologies, such as real-time corrosion sensors and advanced imaging techniques, allow for more precise and efficient detection of corrosion in equipment and infrastructure. These tools enable operators to monitor the condition of materials in real time, providing valuable data that can be used to predict the lifespan of components and optimize maintenance schedules.
As the oil and gas industry continues to evolve, the need for advanced materials and corrosion-resistant technologies will only increase. API corrosion and material certification will continue to play a vital role in ensuring that materials meet the highest standards for performance and reliability. By embracing innovations and maintaining a commitment to rigorous testing and certification, industries can stay ahead of the challenges posed by corrosion and ensure the safety and longevity of their equipment for years to come.
The Role of Materials in the Corrosion Process
Corrosion is not merely an unfortunate consequence of exposure to harsh environments; it is an intricate, multi-faceted process deeply tied to the inherent properties of the materials themselves. The chemistry behind corrosion and material behavior plays a central role in determining the longevity and effectiveness of materials, especially in industries where materials are under constant environmental stress. When exploring the dynamics of corrosion, one cannot ignore the intrinsic relationship between the material's atomic structure and how it interacts with its environment.
At the molecular level, corrosion often occurs when atoms on the surface of a material react with molecules in the surrounding environment. These reactions can lead to the formation of oxides or other compounds that degrade the material. For example, when iron reacts with oxygen in the presence of water, it forms iron oxide, commonly known as rust. This rust is porous, allowing further oxidation to occur beneath the surface, perpetuating the cycle of degradation.
However, the behavior of materials during corrosion is not uniform. Various factors, such as the alloy composition, the presence of certain elements, and even the grain structure, can influence how a material reacts to corrosive agents. For instance, materials that have high levels of chromium, such as stainless steel, are far more resistant to corrosion compared to others due to the formation of a thin, protective oxide layer that prevents further deterioration.
Understanding the characteristics of different materials helps in predicting how they will perform over time when exposed to various corrosive agents. In the case of alloys, corrosion resistance can be fine-tuned by altering the composition to include elements like nickel, copper, or titanium. These materials are often used in industries where equipment is exposed to aggressive chemicals, high temperatures, or extreme moisture conditions. By modifying the alloy’s properties, engineers can ensure that the material performs optimally, even in the most challenging conditions.
In addition to the chemical composition, the physical characteristics of materials, such as surface finish and porosity, also impact corrosion behavior. Materials with rough or porous surfaces are more susceptible to corrosion because they provide more surface area for the corrosive agents to act upon. On the other hand, smoother, denser surfaces typically offer better resistance to corrosion as there is less space for the corrosive elements to penetrate.
Understanding Electrochemical Reactions in Corrosion
The fundamental science behind corrosion is rooted in electrochemistry, specifically the electrochemical reaction between metals and environmental factors. When metals interact with water and oxygen, a series of electrochemical reactions takes place, leading to the deterioration of the material. These reactions can be divided into two key processes: oxidation and reduction.
Oxidation is the process by which a metal loses electrons and is transformed into a positive ion. This occurs when the metal reacts with oxygen or water molecules from the surrounding environment. For instance, iron in the presence of water and oxygen loses electrons, forming iron ions. These ions then react with oxygen to form iron oxide, or rust.
At the same time, reduction takes place at a different location on the metal's surface. Reduction involves the gain of electrons by a substance, and in the case of corrosion, it often occurs when hydrogen ions from water gain electrons to form hydrogen gas. This hydrogen gas can then escape from the surface, leaving behind a weakened, corroded metal structure.
This electrochemical reaction does not happen uniformly across the entire surface of the material. Instead, it occurs at specific sites on the metal, creating what is known as a corrosion cell. Within this cell, the areas where oxidation occurs are known as anodic sites, while the areas where reduction happens are called cathodic sites. The metal’s overall behavior during corrosion is determined by the balance between these anodic and cathodic reactions. The corrosion rate will depend on several factors, including the availability of oxygen, the presence of water or other electrolytes, and the metal's susceptibility to oxidation.
Understanding these electrochemical reactions is essential for developing strategies to mitigate corrosion. By altering the environment around the material or modifying the material itself, engineers can either slow down or completely stop the corrosion process. This can involve using coatings, inhibitors, or more corrosion-resistant materials, as well as controlling factors like temperature, moisture levels, and exposure to corrosive chemicals.
Types of Corrosion and Their Implications for Material Behavior
Corrosion manifests in various forms, each of which presents unique challenges to materials. Understanding the different types of corrosion is critical for choosing the right material and treatment processes. While some forms of corrosion are more obvious and easier to detect, others occur in more subtle ways, causing hidden damage that can remain undetected for long periods.
One of the most common forms of corrosion is uniform corrosion, which occurs evenly across the surface of the material. This type of corrosion is often seen when metals are exposed to oxygen and moisture, leading to the formation of a uniform oxide layer. While uniform corrosion is easier to detect and manage, it can still lead to significant material loss over time if left untreated.
Galvanic corrosion, on the other hand, occurs when two dissimilar metals come into contact in the presence of an electrolyte. In this case, the more reactive metal will corrode, while the less reactive one remains intact. This type of corrosion can be particularly problematic in industries where different metals are used together, such as in plumbing systems, marine applications, or electrical installations.
Crevice corrosion occurs in confined spaces where the flow of oxygen and other corrosive agents is restricted. These crevices can be found in joints, under deposits, or even beneath gaskets, making crevice corrosion difficult to detect. The localized nature of crevice corrosion means that it can cause significant damage to a material even though the rest of the surface may appear unaffected.
Another critical form of corrosion is stress corrosion cracking (SCC), which occurs when materials, particularly metals, are exposed to a corrosive environment while under mechanical stress. This phenomenon is especially dangerous in industries where equipment experiences constant loads or vibrations, as SCC can propagate cracks that compromise the material’s structural integrity. Materials such as stainless steel, aluminum, and high-strength alloys are particularly susceptible to SCC under certain conditions, especially when exposed to chlorides or other aggressive substances.
Understanding these various forms of corrosion helps engineers and material scientists make more informed decisions when selecting materials for specific applications. By considering factors such as exposure to specific chemicals, mechanical stresses, and environmental conditions, engineers can choose materials that are better equipped to withstand these corrosive forces.
The Impact of Corrosion on Industrial Operations and Safety
Corrosion not only threatens the integrity of materials but also poses significant risks to industrial operations and safety. As materials degrade over time, their ability to perform their intended function diminishes. In industries such as oil and gas, chemical processing, and construction, this can have catastrophic consequences if not managed effectively.
In the oil and gas industry, for example, corrosion is a major concern as it can lead to the failure of pipelines, pressure vessels, and other critical components. Corrosion can cause leaks or ruptures in pipelines, leading to the release of hazardous materials, environmental contamination, and even explosions. The costs of repairing or replacing corroded equipment are substantial, and in some cases, the impact on the environment and public health can be severe.
Moreover, the safety of workers is always at risk when corrosion compromises the structural integrity of industrial equipment. Materials that are no longer able to withstand the stresses of operation can fail unexpectedly, leading to accidents or injuries. As such, monitoring corrosion and implementing proactive measures to prevent material degradation is a critical aspect of workplace safety.
Beyond safety concerns, corrosion can also affect the efficiency of industrial operations. As materials degrade, they can become less efficient at conducting heat, electricity, or fluids. This can result in reduced performance, increased energy consumption, and the need for more frequent maintenance. In industries where uptime is critical, such as power generation or manufacturing, corrosion can lead to significant operational disruptions and financial losses.
In response to these challenges, industries have invested heavily in corrosion monitoring and control technologies. Regular inspections, corrosion sensors, and non-destructive testing methods are commonly employed to detect corrosion early, allowing for timely repairs and maintenance. Additionally, advancements in material science have led to the development of corrosion-resistant coatings, alloys, and treatments that can prolong the lifespan of equipment and reduce the likelihood of failure.
Advances in Corrosion Prevention and Material Certification
To combat the growing issue of corrosion, significant advancements have been made in both the prevention of corrosion and the certification of materials. Material certification processes, such as those defined by organizations like the American Petroleum Institute (API), ensure that materials meet specific standards for corrosion resistance and performance under varying environmental conditions.
Material certification involves subjecting materials to a series of rigorous tests to evaluate their resistance to corrosion, stress, and other factors that can affect their performance. These tests are designed to simulate the harsh conditions that materials may encounter during their service life, providing engineers with valuable data about how the materials will behave under different circumstances. By adhering to these certification standards, companies can ensure that they are using the most reliable and durable materials available.
In addition to certification processes, the development of new corrosion-resistant materials has been a significant focus of research and development. Engineers and material scientists have worked to create alloys and coatings that can withstand the most extreme corrosive environments. For example, the development of duplex stainless steels and superalloys has provided industries with materials that offer superior resistance to pitting, crevice corrosion, and SCC.
Furthermore, advances in coating technologies, such as the use of ceramic coatings, polymers, and epoxy-based treatments, have enhanced the ability to protect materials from corrosion. These coatings create a barrier between the material and the corrosive environment, preventing direct contact and thus slowing the corrosion process. In some cases, these coatings are designed to be self-healing, meaning that they can repair themselves if damaged, providing long-lasting protection.
As industries continue to face the challenges posed by corrosion, the importance of material certification and ongoing research cannot be overstated. By continually improving materials and adopting advanced corrosion prevention techniques, industries can reduce the risks associated with material degradation and ensure the safety and efficiency of their operations.
Types of Corrosion and Their Impact on Materials
Corrosion, the gradual degradation of materials due to chemical reactions with their environment, is a phenomenon that poses a significant challenge across various industries. Its effects are often not immediately visible but can lead to substantial damage and failure of structures, machinery, and equipment over time. The types of corrosion that affect materials are varied and complex, each with its own set of characteristics and consequences. This article delves into the primary forms of corrosion, exploring how they affect materials and the mechanisms behind their development. The understanding of these corrosion types plays a pivotal role in ensuring the longevity and reliability of materials used in diverse applications, from construction to manufacturing.
Uniform Corrosion: The Common and Pervasive Threat
Uniform corrosion stands as the most common and easily identifiable form of corrosion. Unlike other types, uniform corrosion occurs evenly across the surface of a material, gradually eroding it over time. This process is most often triggered by environmental factors such as moisture, air, or chemicals that react with the material. When iron, for example, comes into contact with oxygen and moisture, it undergoes a reaction that results in the formation of iron oxide—commonly known as rust. This form of corrosion is often visible to the naked eye, particularly in metals exposed to frequent exposure to humid conditions or water.
While uniform corrosion may appear less severe compared to other types, its effects are far-reaching. The gradual loss of material thickness due to corrosion weakens the integrity of the material, making it more susceptible to failure, especially in high-stress environments. In industrial settings, this can lead to the premature degradation of machinery, pipelines, and structural components. Over time, the progressive thinning of materials under uniform corrosion can cause a critical loss of mechanical strength, necessitating costly repairs or replacements. Preventing and mitigating uniform corrosion involves selecting corrosion-resistant materials and applying protective coatings, as well as performing routine maintenance to ensure early detection and remediation.
Galvanic Corrosion: A Silent Erosion Between Dissimilar Metals
Galvanic corrosion, often referred to as bimetallic corrosion, occurs when two dissimilar metals are in contact with each other in the presence of an electrolyte, such as water or salt. In such an environment, the metals act as electrochemical cells, leading to the accelerated deterioration of the less noble (anodic) metal while the more noble (cathodic) metal remains largely unaffected. This type of corrosion occurs because the electrical potential of different metals varies, creating a situation where the more reactive metal gives up electrons and corrodes faster.
In practice, galvanic corrosion is a significant issue in many industries that rely on the use of multiple metals in a single structure or system. For example, when steel components are used in contact with aluminum, the steel may undergo rapid corrosion, particularly in moist environments. Galvanic corrosion not only compromises the lifespan of materials but also affects the overall functionality of a system. The consequences can be severe, leading to a reduction in performance and even catastrophic failure in critical applications, such as pipelines, bridges, or aerospace components. To mitigate this type of corrosion, it is crucial to select compatible materials that are less likely to form a galvanic couple or use insulating materials to prevent direct contact between dissimilar metals.
Pitting Corrosion: The Silent and Localized Aggressor
Pitting corrosion represents a particularly insidious form of degradation that can be highly destructive yet difficult to detect. Unlike uniform corrosion, which affects a material’s surface evenly, pitting corrosion leads to the formation of small, localized holes or pits in the material. These pits often occur as a result of exposure to chloride ions, such as those found in saltwater or certain industrial chemicals. The localized nature of pitting corrosion makes it more challenging to monitor and prevent because the material surrounding the pits may appear unaffected while the internal structure weakens.
Stainless steel and other alloys exposed to aggressive chemicals or seawater are particularly susceptible to pitting corrosion. The formation of pits can lead to the failure of critical parts, as the pits reduce the material's ability to withstand pressure or external loads. This can be especially dangerous in industries like marine engineering, where components are constantly exposed to saltwater. The development of pitting corrosion often leads to the formation of stress concentrations, which can exacerbate cracking and further accelerate the failure process. To counteract pitting corrosion, materials must undergo rigorous testing to assess their resistance to this form of degradation, and additional protective coatings may be applied to reduce exposure to corrosive elements.
Crevice Corrosion: The Hidden Threat in Confined Spaces
Crevice corrosion is another form of localized corrosion that occurs in narrow and confined spaces, such as the joints between metal components, cracks, or under deposits of material. The key feature of crevice corrosion is that it thrives in areas where stagnant water or chemicals can accumulate, creating an environment conducive to electrochemical reactions. These reactions cause the formation of corrosive pits within the confined area, weakening the material over time.
The challenge with crevice corrosion lies in its stealthy nature. It often occurs in hidden spaces that are difficult to access for inspection or maintenance. For example, the crevices found in the joints of metal plates, bolts, or fasteners can trap moisture, chemicals, and debris, creating the ideal conditions for crevice corrosion to take root. By the time it is detected, the material may have already suffered significant damage. This form of corrosion is a particular concern in industries that use welded metal structures or components with intricate joint designs, such as the aerospace, marine, and chemical processing sectors. To prevent crevice corrosion, it is essential to design components with fewer tight spaces or to apply protective coatings that seal off potential crevices from corrosive agents.
Stress Corrosion Cracking: A Combination of Forces
Stress corrosion cracking (SCC) is a complex form of corrosion that occurs when a material experiences both tensile stress and exposure to a corrosive environment, resulting in the formation of cracks. Unlike other forms of corrosion, SCC is not solely caused by the chemical environment but rather by the interaction between the material’s mechanical stress and the corrosive agents present. This makes stress corrosion cracking particularly dangerous in materials under constant load or stress, such as pipelines, pressure vessels, and structural components.
The combination of stress and corrosion creates a scenario where cracks propagate through the material, leading to the eventual failure of the component. The presence of certain chemicals, such as chlorides, can exacerbate the process by acting as catalysts for crack formation and growth. Stress corrosion cracking can lead to catastrophic failures in critical systems, especially in industries that deal with high-pressure or high-temperature environments. Prevention of SCC requires careful material selection, stress management, and the use of inhibitors or coatings that protect the material from corrosive influences. Monitoring for early signs of SCC is also crucial to preventing significant damage and ensuring the safety of equipment.
Galvanic Corrosion Prevention and Material Certification
One of the most effective ways to prevent galvanic corrosion is through the careful selection of materials. When designing systems that involve multiple metals, engineers can choose materials with similar electrochemical potentials to reduce the likelihood of a galvanic reaction. In addition, protective coatings and insulating barriers can be used to prevent direct contact between dissimilar metals, thereby mitigating the risk of galvanic corrosion. Moreover, industries often rely on material certification programs, such as the American Petroleum Institute (API) corrosion and material certification, to ensure that the materials used in their applications are resistant to various forms of corrosion, including galvanic corrosion.
Material certification programs play a crucial role in ensuring the integrity and durability of materials exposed to corrosive environments. These programs establish rigorous testing standards that materials must meet to prove their resistance to different types of corrosion. By adhering to these standards, industries can select materials that are better equipped to withstand the corrosive forces they will encounter, ultimately enhancing the longevity and performance of their systems. The process of material certification is an essential step in reducing the risks associated with corrosion and ensuring the safety and reliability of industrial infrastructure.
In the context of industrial processes, especially within the oil and gas sector, the selection of appropriate materials is a paramount consideration. The material chosen for any given application must exhibit resilience against various environmental stresses, including corrosion, mechanical strain, and extreme temperatures. The American Petroleum Institute (API) plays a critical role in material selection, providing industry-specific guidelines that ensure materials meet the rigorous demands of oilfield operations. API standards serve as the benchmark for assessing material performance, ensuring that the materials chosen for use in pipelines, pumps, and tanks are fit for purpose.
These standards not only foster operational safety but also extend the lifespan of equipment, reducing maintenance costs and minimizing the risk of catastrophic failures. By adhering to API guidelines, companies can rest assured that their chosen materials are up to the task, capable of withstanding the unique challenges posed by the harsh environments typical of the oil and gas industry. API's influence on material selection has revolutionized the approach to manufacturing, emphasizing long-term durability, environmental compatibility, and overall operational efficiency.
API Standards and Their Core Principles in Material Selection
API standards are designed with a core set of principles that guide the selection, testing, and certification of materials for oil and gas applications. These guidelines focus primarily on ensuring that materials maintain their structural integrity when subjected to severe operational conditions such as high pressures, extreme temperatures, and aggressive chemical exposure. The standards set forth by API aim to minimize the risk of failure, safeguard human life, and preserve environmental integrity, making them a fundamental aspect of industry practice.
One of the foundational principles of API standards is corrosion resistance. Many materials used in the oil and gas sector must withstand exposure to aggressive chemicals, seawater, or acidic environments. API standards address these concerns by establishing guidelines for material selection based on a material's ability to resist specific types of corrosion, such as uniform, pitting, and crevice corrosion. In this regard, API standards emphasize the importance of material testing under simulated environmental conditions, ensuring that selected materials perform optimally under real-world stresses.
Another key principle outlined in API standards is mechanical performance. API guidelines stipulate that materials must not only resist corrosion but also maintain their mechanical properties, such as tensile strength, hardness, and ductility throughout their service life. This ensures that the material can bear the weight, pressure, and stress associated with oil and gas production and transportation processes.
Corrosion Resistance: A Core Element of Material Selection
Corrosion is perhaps the most significant challenge facing materials used in the oil and gas industry. Exposure to harsh chemicals, extreme temperatures, and saline environments can lead to the degradation of materials, resulting in leaks, equipment failure, and even catastrophic accidents. For this reason, corrosion resistance is one of the most important factors considered during material selection. API standards provide clear and comprehensive guidelines for assessing how materials respond to different types of corrosion.
Materials such as stainless steel, which are known for their inherent corrosion resistance, are often subjected to rigorous testing as part of the certification process. Stainless steel’s resistance to corrosion is tested in various forms, including its ability to resist crevice corrosion, pitting corrosion, and stress corrosion cracking. Other materials, such as carbon steel, are evaluated for their resistance to uniform corrosion, particularly in environments where moisture or acidic conditions may prevail. By adhering to API standards, manufacturers and engineers can ensure that materials chosen for critical infrastructure are capable of withstanding the corrosion challenges inherent in the oil and gas industry.
API guidelines also provide recommendations for addressing issues related to localized corrosion. For example, pitting corrosion occurs when small pits or cavities form on a material's surface, which can eventually compromise its structural integrity. API's standards recommend various materials that are particularly resistant to pitting, such as certain alloys of stainless steel, and provide guidelines for their selection based on specific operational conditions.
Mechanical Properties and Structural Integrity in Harsh Conditions
While corrosion resistance is a central focus of API standards, the mechanical properties of materials are equally important in ensuring that they perform adequately in extreme conditions. In many oil and gas applications, materials are subjected to high pressures, temperatures, and mechanical stress that can affect their strength and resilience. API standards assess materials not only for their ability to resist corrosion but also for their mechanical properties, such as tensile strength, hardness, and impact toughness.
Tensile strength is an essential property that indicates how much force a material can withstand before breaking. Materials used in oil and gas applications must have sufficient tensile strength to handle the immense pressures associated with fluid transport, drilling, and other industrial processes. API guidelines provide comprehensive testing protocols to ensure that materials can endure the high-stress environments found in the field.
Similarly, hardness is another critical mechanical property. It measures a material’s ability to resist deformation under stress. Hardness tests are integral to the API certification process, as they help determine how well a material can resist wear, abrasion, and other forms of mechanical damage over time. Additionally, the ductility of a material is also assessed. Ductility refers to a material’s ability to stretch or deform without breaking, which is crucial in situations where materials may need to withstand dynamic stresses or movements.
API standards ensure that the materials chosen for infrastructure, such as pipelines, tanks, and valves, have adequate mechanical properties to perform in the challenging environments of the oil and gas industry. Without the rigorous evaluation of these characteristics, the risk of material failure would be substantially higher, potentially leading to costly and dangerous operational disruptions.
Chemical Composition and Its Impact on Material Performance
The chemical composition of materials plays a crucial role in determining their performance and resistance to corrosion in oil and gas environments. API standards require that materials meet specific chemical requirements to ensure their reliability and durability. The presence of alloying elements, such as chromium, nickel, and molybdenum, can significantly enhance the material's resistance to corrosion and its mechanical properties.
For instance, the addition of chromium to steel increases its resistance to oxidation and corrosion, particularly in high-temperature environments. Nickel, on the other hand, improves a material's resistance to pitting corrosion and enhances its ability to perform in acidic conditions. Molybdenum is added to increase resistance to crevice corrosion and improve the material’s strength at high temperatures.
API certification involves a detailed analysis of a material’s chemical composition to ensure that it meets the required standards for specific operational conditions. This analysis ensures that the material has the necessary elements in the correct proportions, contributing to its overall performance and reliability. The chemical composition of a material must be carefully controlled to prevent the formation of weak points that could lead to failure under stress or corrosion.
By following API's chemical composition guidelines, manufacturers can produce materials that are not only resistant to environmental stresses but also capable of maintaining their structural integrity over the long term. This is crucial for preventing unexpected material failures that could result in significant downtime, expensive repairs, and safety hazards.
Ensuring Long-Term Durability and Cost-Effectiveness
The importance of adhering to API standards in material selection extends beyond just ensuring operational safety and performance. One of the most significant benefits of API certification is the potential for long-term cost savings. By selecting materials that are specifically designed to withstand the harsh conditions of the oil and gas industry, companies can reduce the frequency of repairs, replacements, and maintenance interventions.
Materials that meet API standards are built to last, providing a high level of reliability even in extreme conditions. This durability helps prevent equipment breakdowns, reducing the need for costly repairs and replacements. Furthermore, by avoiding the premature failure of materials, companies can minimize production downtime, which directly contributes to the overall profitability and efficiency of operations.
The cost-effectiveness of using API-certified materials is particularly evident when considering the long-term performance and operational reliability they offer. Although materials that meet API standards may come with a higher upfront cost, the benefits they provide in terms of reducing maintenance costs, extending the lifespan of equipment, and avoiding costly disruptions make them a smart investment. In the competitive and high-stakes environment of oil and gas production, making the right material choice can have a significant impact on a company’s bottom line.
Impact of API Standards on Operational Safety and Environmental Protection
One of the key drivers behind the development of API standards is the need to protect both human life and the environment. In the oil and gas industry, operational accidents resulting from material failures can have catastrophic consequences, including loss of life, environmental damage, and significant financial losses. By adhering to API standards in material selection, companies can mitigate these risks and help ensure that their operations are safe and environmentally responsible.
API’s emphasis on corrosion resistance, mechanical strength, and chemical composition plays a pivotal role in preventing material failures that could lead to hazardous situations. For example, the use of corrosion-resistant materials in pipelines and tanks helps reduce the risk of leaks or spills, which can lead to devastating environmental consequences. Additionally, materials that meet API standards are less likely to experience catastrophic failures, which can result in unsafe working conditions for employees.
Furthermore, by ensuring that materials meet the necessary standards for structural integrity and chemical compatibility, API guidelines help companies minimize their environmental footprint. The oil and gas industry is under increasing scrutiny for its environmental impact, and the use of certified materials is one way to demonstrate a commitment to sustainability and responsible resource management.
In this way, API standards not only help improve operational efficiency and reduce costs but also promote a culture of safety and environmental stewardship within the industry. Adopting these standards is an essential step toward ensuring that oil and gas operations are conducted with the utmost consideration for both human and ecological well-being.
Corrosion is one of the most significant challenges faced by industries that operate in harsh environmental conditions. Whether in maritime, chemical, or construction sectors, understanding and mitigating corrosion is critical for maintaining the integrity, safety, and longevity of materials and equipment. In this comprehensive discussion, we will delve into the practices and strategies that help ensure corrosion resistance in materials. Through a well-structured approach, we will uncover methods that not only prevent corrosion but also protect investments, reduce downtime, and maintain operational efficiency.
Material Selection: The First Line of Defense
The selection of materials plays a pivotal role in preventing corrosion. Corrosion-resistant materials are specifically engineered to withstand the effects of chemical reactions and environmental degradation. These materials are typically alloys, composites, or metals that possess intrinsic corrosion resistance. Stainless steel, for example, is widely known for its resistance to rust and pitting corrosion due to its chromium content, which forms a protective oxide layer on the surface.
In some environments, selecting materials based on their electrochemical properties is crucial. The galvanic series, which ranks metals according to their electrochemical potential, can be an essential tool in avoiding galvanic corrosion—a type of corrosion that occurs when two different metals are in electrical contact in the presence of an electrolyte. By selecting materials that fall closer together in the galvanic series, one can reduce the likelihood of galvanic corrosion.
Beyond stainless steel, certain coatings and surface treatments can significantly enhance a material’s ability to resist corrosion. For example, materials that are prone to rusting, like carbon steel, can be coated with zinc, a process known as galvanization. This zinc layer acts as a sacrificial anode, corroding first and thereby protecting the steel beneath it. Choosing the right combination of base material and protective coatings can be a game-changer in preventing corrosion-related failures.
The Role of Coatings and Surface Treatments
Coatings and surface treatments represent one of the most effective ways to combat corrosion. The application of protective coatings forms a barrier between the metal and its environment, preventing harmful substances from coming into contact with the material’s surface. These coatings can range from simple paints to more advanced systems like epoxy coatings or polymer-based films.
Epoxy coatings, in particular, are known for their excellent adhesion and chemical resistance. These coatings are often applied to pipelines, tanks, and other metal surfaces that are exposed to aggressive environments such as acidic or saline waters. Polyurethane coatings also offer a high level of durability and resistance to ultraviolet light, making them ideal for outdoor applications where exposure to sunlight could degrade the material.
Another promising development is the use of nanotechnology in coatings. Nanocoatings are designed to provide ultra-thin, highly durable, and corrosion-resistant films that are applied at the molecular level. These coatings not only protect against corrosion but can also provide self-healing properties, where the coating repairs itself if it becomes scratched or damaged. This innovative approach offers a longer-lasting solution to corrosion, reducing the frequency and cost of repairs and replacements.
It’s important to note that applying coatings requires careful consideration of surface preparation. For the coating to adhere properly and provide the expected level of protection, the surface must be thoroughly cleaned, degreased, and sometimes even roughened. Surface treatments like sandblasting or acid etching are often necessary to ensure a strong bond between the substrate and the coating.
Regular Inspection and Monitoring Systems
Even with the most corrosion-resistant materials and coatings, corrosion can still occur over time. Regular inspection and monitoring are essential to catch the early signs of corrosion before they lead to equipment failure or significant damage. Routine inspections typically include visual checks, but more advanced techniques can also be employed to detect hidden or internal corrosion.
Ultrasonic testing (UT) is one such non-destructive testing method. UT uses high-frequency sound waves to detect changes in material thickness, which can indicate the presence of corrosion or other forms of degradation. This method is especially useful for inspecting pipelines, storage tanks, and other critical infrastructure that may not be easily accessible for visual inspection.
Eddy current testing (ECT) is another non-invasive technique that detects surface cracks and corrosion in conductive materials. By generating a magnetic field and observing the resulting eddy currents, technicians can identify anomalies in the material that suggest corrosion or other structural issues.
In some cases, companies may also use corrosion monitoring systems that involve the installation of sensors or probes within the equipment to continuously measure the rate of corrosion. These real-time monitoring systems help detect corrosion early, allowing for timely intervention and maintenance. Integrating corrosion monitoring into regular maintenance routines can significantly extend the lifespan of equipment, reduce unplanned downtime, and improve overall operational efficiency.
The Impact of Environmental Factors
Corrosion does not occur in a vacuum. It is highly influenced by environmental conditions such as temperature, humidity, salinity, and the presence of certain chemicals. Industries operating in coastal areas, for instance, face a higher risk of corrosion due to the presence of saltwater, which accelerates the corrosive process. Similarly, chemical plants often deal with corrosive substances like acids or alkalis that can eat away at metals and other materials.
Understanding these environmental factors and their effects on materials is essential for making informed decisions about corrosion prevention. For example, materials exposed to high humidity may require coatings that are resistant to moisture absorption, while those in saline environments may benefit from more robust coatings or sacrificial anodes to protect against salt-induced corrosion.
In some cases, modifying the environment can also help reduce the impact of corrosion. In industrial settings where equipment is exposed to corrosive agents, controlling factors like temperature and humidity can slow down the rate of corrosion. This can be achieved through the use of controlled atmospheres, dehumidifiers, or even corrosion inhibitors in the air or water supply.
By understanding the environmental conditions at play, companies can select materials, coatings, and maintenance practices that are tailored to their specific needs. This personalized approach helps minimize the risk of corrosion and prolong the lifespan of critical infrastructure.
Corrosion Inhibitors: A Chemical Solution
Corrosion inhibitors are chemicals that are used to slow or prevent the corrosion process. These inhibitors work by forming a protective layer on the surface of the material, preventing the corrosive agents from coming into contact with the metal. Inhibitors are commonly used in systems like cooling towers, pipelines, and boilers, where corrosion can be a persistent issue.
There are two primary types of corrosion inhibitors: anodic and cathodic inhibitors. Anodic inhibitors work by forming a protective oxide layer on the metal surface, reducing the rate of oxidation. Cathodic inhibitors, on the other hand, reduce the cathodic reaction in the electrochemical process, slowing down the overall rate of corrosion.
The application of corrosion inhibitors can be highly effective in industries where corrosion is a constant threat. For example, in the oil and gas sector, corrosion inhibitors are often added to the fluids circulating in pipelines to protect against the corrosive effects of crude oil, natural gas, and water. These inhibitors can be used in conjunction with other preventive measures, such as coatings and material selection, to provide a comprehensive corrosion control strategy.
However, it’s essential to monitor the concentration of corrosion inhibitors to ensure their effectiveness. Too little inhibitor may not provide sufficient protection, while too much can lead to environmental concerns or increased operational costs. Therefore, regular testing and optimization of inhibitor levels are essential for maintaining the ideal balance and preventing corrosion effectively.
Maintenance and Replacement: Staying Ahead of Corrosion
Despite the best efforts in material selection, coatings, and inhibitors, corrosion will eventually take its toll on materials over time. Regular maintenance is essential for identifying corrosion early and addressing it before it leads to catastrophic failures. This includes routine inspections, as well as cleaning, lubrication, and the application of additional protective coatings as needed.
In some cases, parts of the equipment may need to be replaced entirely if they have been severely corroded or damaged. Timely replacement helps prevent further degradation and ensures the continued safe operation of the equipment. However, replacement should not be viewed as a last resort; proactive replacement based on regular inspections can help minimize downtime and avoid costly emergency repairs.
Additionally, adopting a preventive maintenance schedule that incorporates corrosion monitoring systems can help identify areas of concern early on. Predictive maintenance techniques, which use data analytics and real-time monitoring, can help forecast when corrosion will reach a critical threshold, allowing for repairs or replacements to be scheduled at the most convenient times.
By combining regular maintenance, monitoring, and a proactive approach to material selection and corrosion prevention, industries can stay ahead of the inevitable effects of corrosion, extending the life of their infrastructure and reducing overall costs.
Conclusion
Through a combination of proper material selection, protective coatings, routine inspections, and the strategic use of corrosion inhibitors, industries can significantly reduce the risk of corrosion-related failures. Understanding the various factors that contribute to corrosion, including environmental conditions and chemical reactions, is essential for developing a comprehensive corrosion resistance strategy. Furthermore, embracing new technologies like nanocoatings, corrosion monitoring systems, and predictive maintenance ensures that equipment remains safe, efficient, and reliable for years to come.
By implementing these best practices and staying ahead of potential issues, companies can safeguard their investments, optimize performance, and enhance the safety and longevity of their assets.
Frequently Asked Questions
How does your testing engine works?
Once download and installed on your PC, you can practise test questions, review your questions & answers using two different options 'practice exam' and 'virtual exam'. Virtual Exam - test yourself with exam questions with a time limit, as if you are taking exams in the Prometric or VUE testing centre. Practice exam - review exam questions one by one, see correct answers and explanations).
How can I get the products after purchase?
All products are available for download immediately from your Member's Area. Once you have made the payment, you will be transferred to Member's Area where you can login and download the products you have purchased to your computer.
How long can I use my product? Will it be valid forever?
Pass4sure products have a validity of 90 days from the date of purchase. This means that any updates to the products, including but not limited to new questions, or updates and changes by our editing team, will be automatically downloaded on to computer to make sure that you get latest exam prep materials during those 90 days.
Can I renew my product if when it's expired?
Yes, when the 90 days of your product validity are over, you have the option of renewing your expired products with a 30% discount. This can be done in your Member's Area.
Please note that you will not be able to use the product after it has expired if you don't renew it.
How often are the questions updated?
We always try to provide the latest pool of questions, Updates in the questions depend on the changes in actual pool of questions by different vendors. As soon as we know about the change in the exam question pool we try our best to update the products as fast as possible.
How many computers I can download Pass4sure software on?
You can download the Pass4sure products on the maximum number of 2 (two) computers or devices. If you need to use the software on more than two machines, you can purchase this option separately. Please email sales@pass4sure.com if you need to use more than 5 (five) computers.
What are the system requirements?
Minimum System Requirements:
- Windows XP or newer operating system
- Java Version 8 or newer
- 1+ GHz processor
- 1 GB Ram
- 50 MB available hard disk typically (products may vary)
What operating systems are supported by your Testing Engine software?
Our testing engine is supported by Windows. Andriod and IOS software is currently under development.